Figure 9
Mesoscopic irreversible thermodynamics of morphological evolution kinetics of helical conformation in bioproteins ‘DNA’ under the isothermal isobaric conditions
Tarik Omer Ogurtani* and Ersin Emre Oren
Published: 11 March, 2020 | Volume 4 - Issue 1 | Pages: 009-019
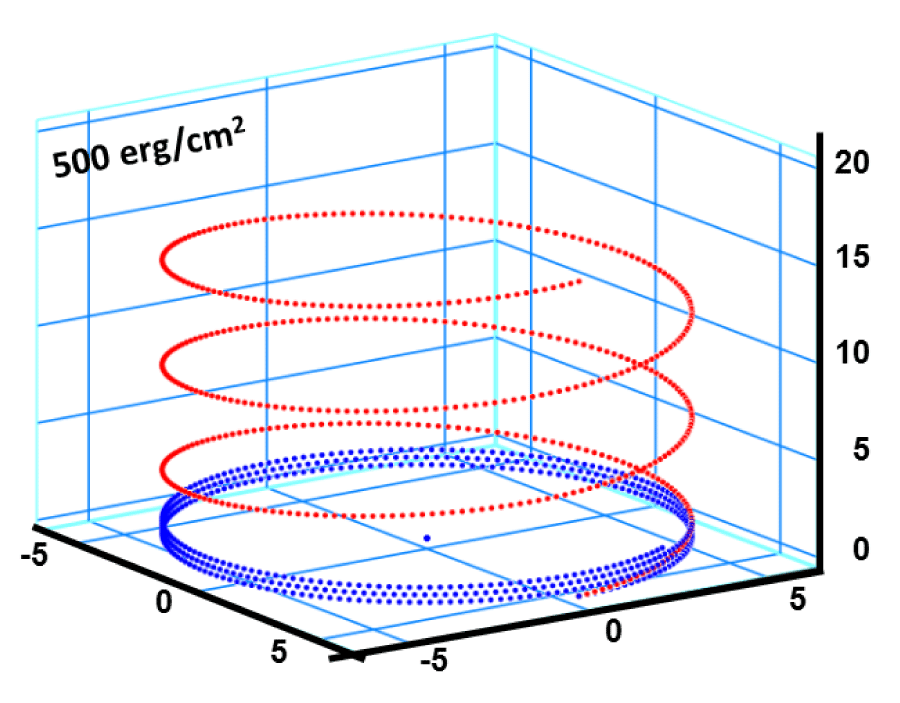
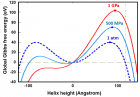
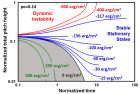
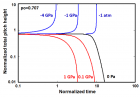
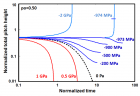
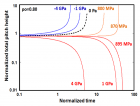
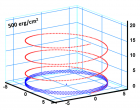
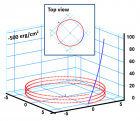
Figure 9:
Helical conformation of α - peptide at the stationary state is illustrated by (red dotted line), which has pitch height 5.4Ao, and radius 6Ao and 3 rings. When it is exposed to the spontaneous relaxation under the positive interfacial Gibbs free energy of gs = +500 erg/cm2 [= 1.677GPa] under 1 ATM pressure (isobaric) then the unfolding takes place (blue line), which is found to be 86.4% of the full transition, having Θ = 3.077 cycle.
Read Full Article HTML DOI: 10.29328/journal.abse.1001008 Cite this Article Read Full Article PDF
More Images
Similar Articles
-
Peptide-based antifouling aptasensor for cardiac troponin I detection by surface plasmon resonance applied in medium sized Myocardial InfarctionJen-Tsai Liu*,Yi Wu,Jia Xin Che,Shwu Jen Chang,Ching-Jung Chen*. Peptide-based antifouling aptasensor for cardiac troponin I detection by surface plasmon resonance applied in medium sized Myocardial Infarction. . 2020 doi: 10.29328/journal.abse.1001007; 4: 001-008
Recently Viewed
-
A rare case of giant ovarian serous cystadenoma presenting as psuedo-meigs syndromeRichmond Ronald Gomes*,Sayeda Noureen,Habiba Akhter. A rare case of giant ovarian serous cystadenoma presenting as psuedo-meigs syndrome. Clin J Obstet Gynecol. 2021: doi: 10.29328/journal.cjog.1001078; 4: 010-014
-
Three visionaries for HRTJoseph Loze Onwude*. Three visionaries for HRT. Clin J Obstet Gynecol. 2021: doi: 10.29328/journal.cjog.1001077; 4: 007-009
-
The Utility of Acupuncture in Sports Medicine: A Review of the Recent LiteratureMichael Malone*. The Utility of Acupuncture in Sports Medicine: A Review of the Recent Literature. J Sports Med Ther. 2017: doi: 10.29328/journal.jsmt.1001004; 2: 020-027
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024: doi: 10.29328/journal.acr.1001099; 8: 075-077
-
Sex after Neurosurgery–Limitations, Recommendations, and the Impact on Patient’s Well-beingMor Levi Rivka*, Csaba L Dégi. Sex after Neurosurgery–Limitations, Recommendations, and the Impact on Patient’s Well-being. J Neurosci Neurol Disord. 2024: doi: 10.29328/journal.jnnd.1001099; 8: 064-068
Most Viewed
-
Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth EnhancersH Pérez-Aguilar*, M Lacruz-Asaro, F Arán-Ais. Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers. J Plant Sci Phytopathol. 2023 doi: 10.29328/journal.jpsp.1001104; 7: 042-047
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001099; 8: 075-077
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Pediatric Dysgerminoma: Unveiling a Rare Ovarian TumorFaten Limaiem*, Khalil Saffar, Ahmed Halouani. Pediatric Dysgerminoma: Unveiling a Rare Ovarian Tumor. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001087; 8: 010-013
-
Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative reviewKhashayar Maroufi*. Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative review. J Sports Med Ther. 2021 doi: 10.29328/journal.jsmt.1001051; 6: 001-007

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."