About Middle East Technical University
Middle East Technical University
Articles by Middle East Technical University
Mesoscopic irreversible thermodynamics of morphological evolution kinetics of helical conformation in bioproteins ‘DNA’ under the isothermal isobaric conditions
Published on: 11th March, 2020
OCLC Number/Unique Identifier: 8570215259
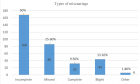
The morphological evolution kinetics and instabilities of alpha helical peptide 3.613, which involves large amount of stored torsional elastic deformation energy (3-40 eV/molecule), is formulated by the variational method based on the connection between the rates of internal entropy production and the changes in the global Gibbs free energy, assuming that one has isobaric irreversible processes under the isothermal conditions. The present mesoscopic nonequilibrium thermodynamic approach relies on the fact that the global Gibbs free energy of helical conformation involves not only the bulk Gibbs free energy of the amino-acid back bone structure but also the interfacial Gibbs free energy of the enclosing cylindrical shell or the cage associated with the side-wall molecular branches, and their interactions with the immediate surroundings. The proposed variational analysis applied directly on the proposed macro-model has furnished a nonlinear integral equation in terms of the normalized and scaled internal and external variables. This allows us to track down the motion of the total pitch height of the alpha polypeptide along the well-defined trajectories in the displacement-time space, dictated not only by the initial configuration of the helix but also through the gradients of the global Gibbs free energy of the strained helical conformation as the main driving force. In the negative manifold, there is a well-defined region below the dynamic instability regime, in which the helical conformation can evolve towards the nonequilibrium stationary states by expanding, or contracting, depending upon whether the interfacial free energy and/or the applied stress system are below or above the well-defined thresholds level dictated by the initial pitch height. The highest life time may be realized along that trajectory, which follows up the threshold level of the interfacial specific Gibbs free energy, which is gs = -315 erg/cm2. In the upper region of the negative manifold, the helical conformations are driven by the very large applied uniaxial tension or the negative pressure induced by the thermal expansion, in the range of p > 1GPa and/or the strong negative interfacial free energies [3-4 pH] or their combinations, they show strong kinematic instabilities, which can cause not only the accelerated unfolding phenomenon but also cause large extensions that end up with the catastrophic decimations by ruptures and fragmentations. In the positive manifold, the aging behavior of the polypeptide follows up a S-shape path having rather speedy aging behavior compared to the negative manifold, which is separated from by a well-defined boundary, which represents the isochoric path having longest relaxation times, which can be achieved with great stability. Finally, one could attempt to estimate the upper limit of the relaxation time of aging for the modern hominin, from samples of exceptional preservations, relying on the present nonequilibrium theory as well as on the very limited knowledge on the post-mortem DNA and the present pitch heights of the modern hominin, which is found to be about 25,840 yrs, with a life expectation of 451,800 yrs. These figures are very close to those calculated for Neanderthals (SH), which are found to be 31,820 yrs and 499,100 yrs, respectively.
Enlarged Curvature, Torsion and Torque in Helical Conformations and the Stability and Growth of α-Peptide under the Isochoric and Isobaric Conditions: Variatonal Optimization
Published on: 7th October, 2024
The torsional deformation behavior of an elastic bar with a circular cross-section was investigated by applying invariant dyadic analysis, where the small finite displacement functions advocated by Saint-Venant (1855) were fully employed. It was found that the previously overlooked circumferential shear force field generated by pure torsion on the side walls of a bar produces an unusual torque term induced by the skew-symmetric part of the deformation tensor and exhibits quadratic length dependence along the z-axis of the bar. The adaptation of this torque term for a helical conformation of α-peptides creates moments acting on the circular cross-sections and is directed along the surface normal of circular cross-sections, which coincides with the tangent vector of the helix. The projection of this torque along the z-axis of the helix varies quadratically with the azimuthal angle. The radial component of the unusual torque, which also lies along the principal normal vector of the helix, starts to perform a precession motion by tracking a spiral orbit around the z-axis, whereas its apex angle decreases asymptotically with the azimuthal angle and finally reaches a finite value depending on the height of the helix along the z-axis. The ordinary torque terms, which are also deduced from the self- and anti-self-conjugate parts of the deformation tensor, have magnitudes half that of the full torque term reported in the literature. The present results were applied to the helical conformation of α-peptides designated by {3.611} to show that the mechanical stability of strained open-ended helical conformations can be successfully achieved by spontaneous readjustments of the surface and bulk Helmholtz free energies under isothermal isochoric conditions. It has been demonstrated that the main contribution to the mechanical stability of α-peptide 3.611 cannot come alone from the electrostatic dipole-dipole interaction potential of the anti-align excess dipole pairs but also from the surface Helmholtz free energy, which is characterized by a binding free energy of -15.5 eV/molecule (-32.56 Kcal/mole) for an alpha-peptide composed of 11 amino acid residues with a critical arc length of approximately 10 nm, assuming that the shear modulus is G = 1GPa and the surface Helmholtz specific free energy density is fs = 800 erg/cm2. This result was in excellent agreement with the experimental observations of the AH-1 conformation of (Glu)n Cys at pH 8. The present theory indicates that only two excess permanent anti-align dipole pairs for one α-Helical peptide molecule is requirement to stabilize the whole secondary structure of the protein that is exposed to heavy torsional deformation during the folding processes which amounts to 7.75 eV/molecule stored electrostatic energy compared to the interfacial Helmholtz free energy of -23.25 eV/molecule, which is exposed to hydrophobic environments.

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."